SubQ BIOLOGICS™
Innovative Nano/Microparticle Injectable Technology
- Modulate solubility and dissolution properties of biologics
- Extended-release PK profiles
- Improved bioavailability and injection site tolerability
- Improved biologics stability
Applications
- Peptide
- Protein
- Antibody
- Oligonucleotide (RNAi, siRNA, DNA, mRNA)
- Mucopolysaccharide
Formulation & Process
- GRAS excipients with varying physicochemical properties
- Formulation with 35 to 70% active drug (remainder GRAS)
- Efficient manufacturing process with >80% yield
SubQ BIOLOGICS™ FORMULATION
| CHARGED AND HYDROPHILIC COUNTERIONS | CHYDROPHOBIC COUNTERIONS |
| Glutamate | Deoxycholate |
| Glycinate | Oleate |
| Lysinate | Stearate |
| Citrate | Nicotinate |
| Succinate | Palmitate |
| Tartrate | Octanoate |
+
| COMPLEXING AGENTS |
| Bivalent Metal Ions (Zn2+, Ca2+, etc…) |
Nano/Microparticles
- Composition falls into 3 major categories
- Selection based on properties of the biologic
- Selected from handbook of pharmaceutical excipients and GRAS list
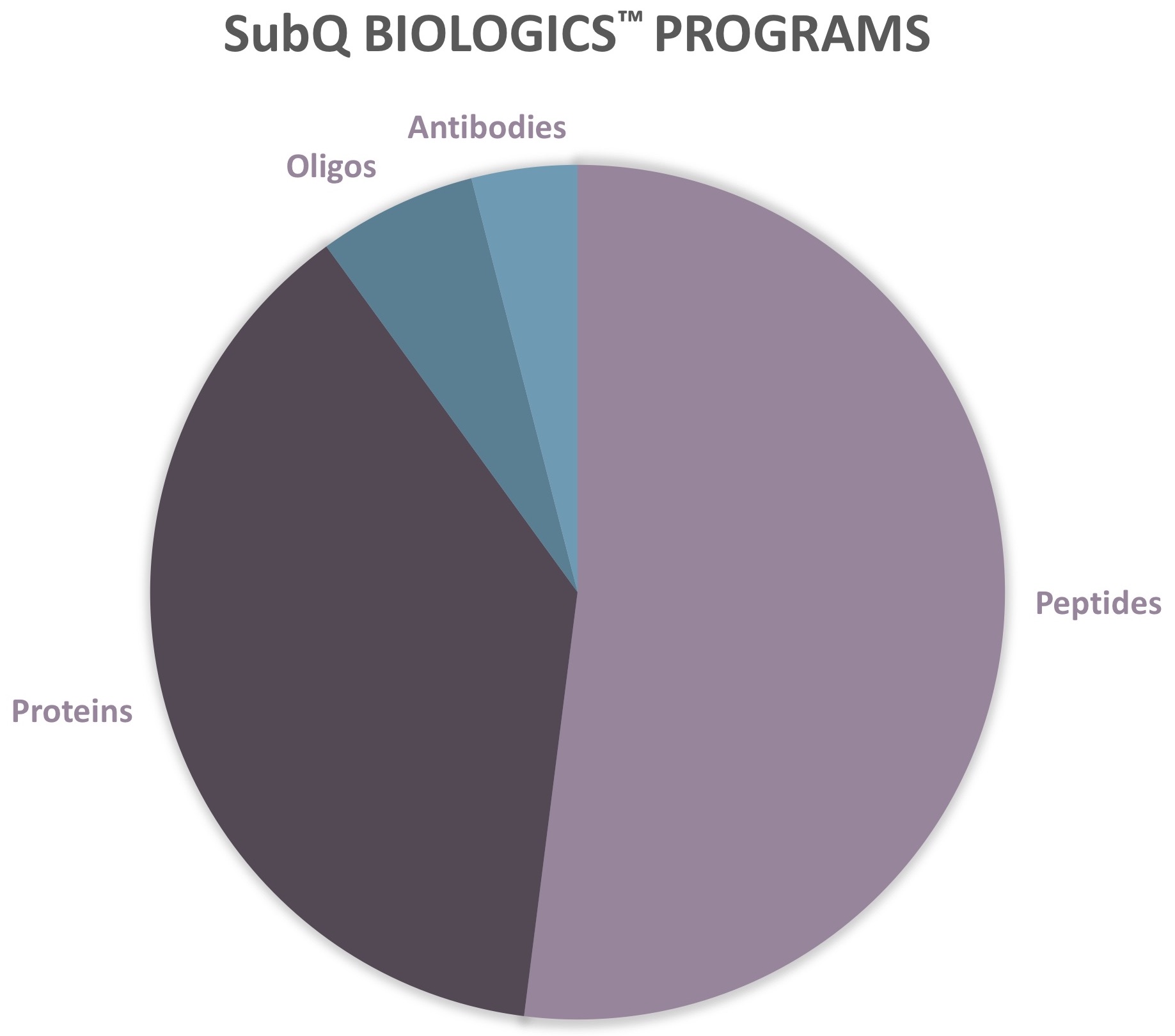
SubQ BIOLOGICS™ PROGRAMS
Peptides
- Numerous partnerships− public and private
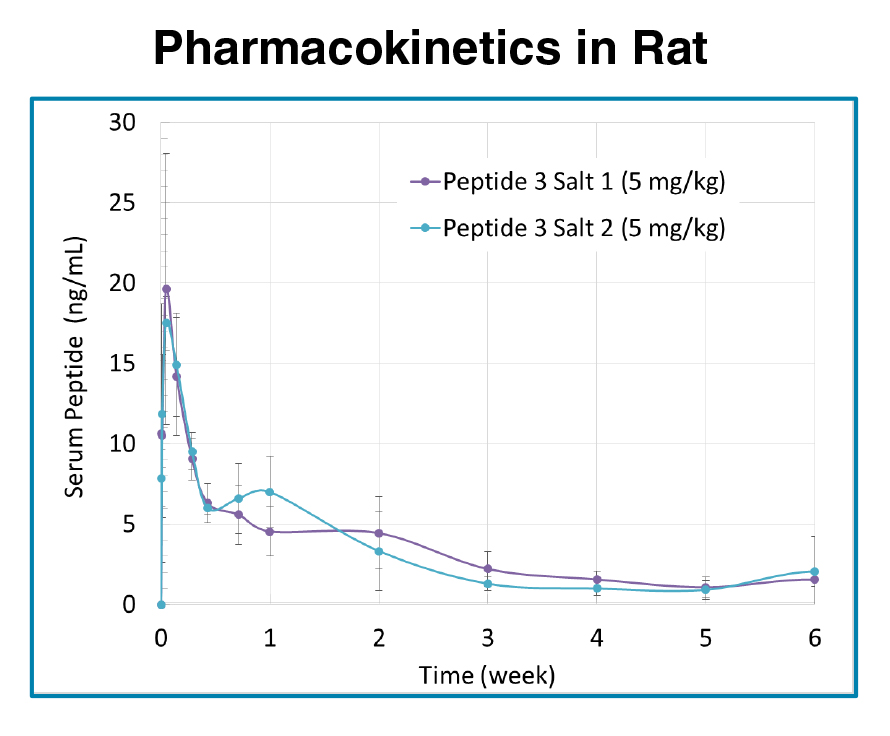
- 1 to 6 weeks PK (rat)
- Improved injection site reaction
Proteins
- Up to 1 week PK (rat)
- Improved injection site reaction
Oligonucleotides
- ASO for T1DM reversal and CNS
- Mouse tox study data
Antibodies
- High concentration solution / suspension
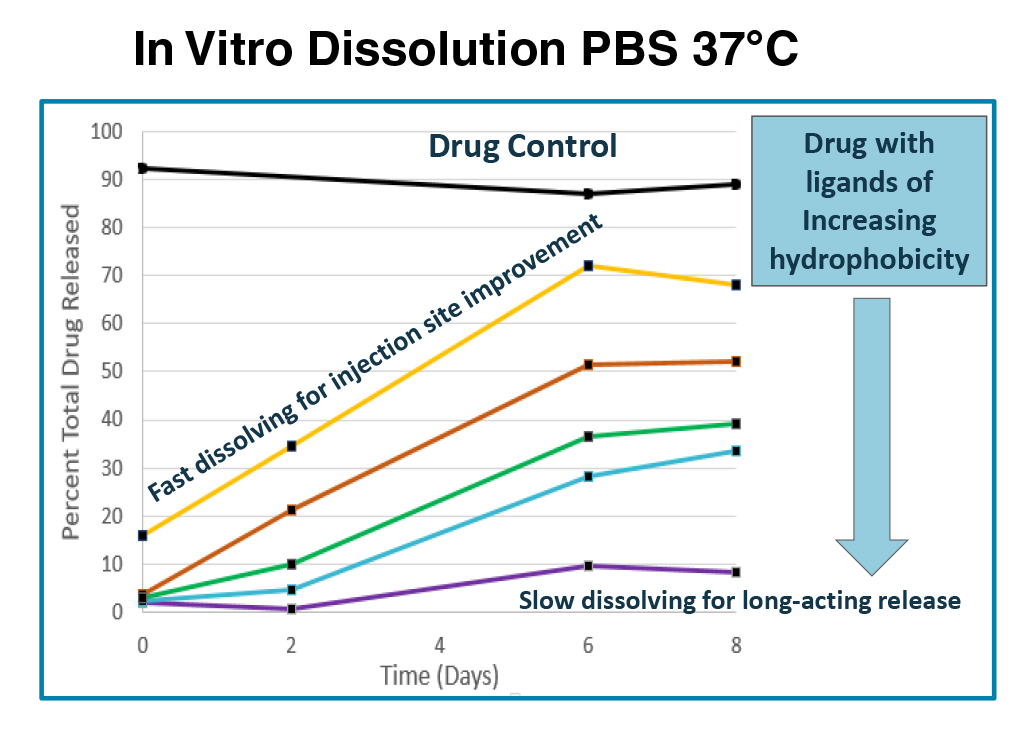
PEPTIDE IVR and PK DATA