PROPEL BIOLOGICS™ JetCAP™
Revolutionary Oral Capsule Device for Precision Delivery of Biologics
- Needle-free fluid transport to gut wall
- 10 - 30 mg per dose
- Systemic delivery comparable to SC
- Targeted region-specific GI delivery
- Standard pharmaceutical components
- Enteric coated - GRAS excipients
- Low cost of goods
- Liquid-fill manufacturing
*Device size is comparable to 000 Capsule Size
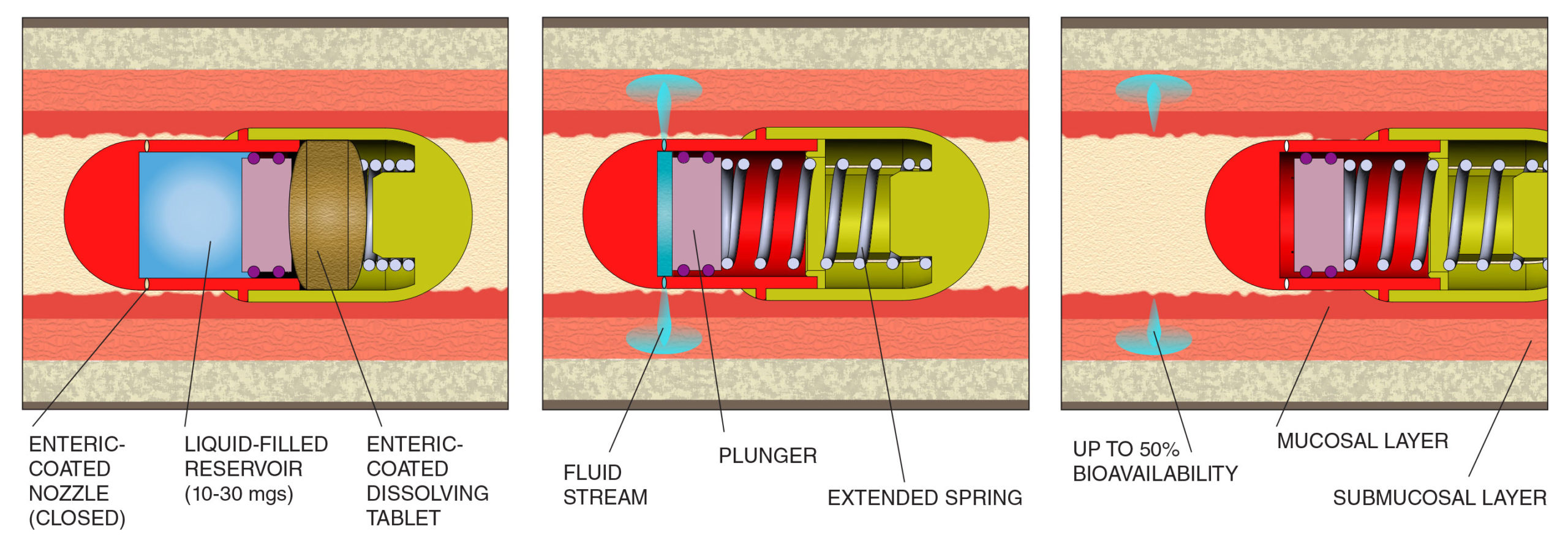
ACTUATION: At a defined pH in the GI tract, enteric coated tablet and nozzle plugs dissolve, preparing the device for delivery of drug.
DEPLOYMENT: Dissolution of the tablet releases the spring, which pushes the plunger, delivering drug from the liquid-filled reservoir to the gut wall.
ELIMINATION: The fluid stream is delivered
at the defined depths to the gut wall. The expended capsule passes harmlessly in unopened state.
High drug delivery capacity
Common pharmaceutical components
Liquid-filled reservoir
- 10 to 30 mg per day delivery
- Potential for les frequent administration
- Ultra-long acting oral product
- Injection molded parts
- Plunger and nozzle
- Tablet actuation mechanism
- Solution or suspension formulation
- Rapidly adaptable from injectable product
- Long-acting particulate suspensions
| JetCAP™ | RaniPill™ | MIT SOMA | |
|---|---|---|---|
| High Dose 10-30 mgs | ✓ | ||
| Needle-Free | ✓ | ||
| Extended-release Formulation | ✓ | ||
| Standard Pharma Components | ✓ | ||
| Liquid Fill Manufacture | ✓ | ||
| Targeted GI Delivery | ✓ | ✓ | |
| High Bioavailability | ✓ | ✓ | ✓ |
| Oral Delivery of Biologics | ✓ | ✓ | ✓ |

COP capsule with nozzle
- Stainless prototype for initial testing of forces
- Cyclic olefin polymer for use in humans / preclinical studies
- COP well known to be compatible to biologics fluids
- Capsule is 000 in size and is excreted
| Dose Range | Delivery Method | Formulation | Status | |
|---|---|---|---|---|
| Baywind Bio JetCAP™ | 10-30 mgs (0.3 ml) | Needle free liquid transport to gut wall | Solution or suspension | Preclinical dog study preparations |
| Progenity Intellicap™ | 10-30 mgs (0.3 ml) | Controlled liquid delivery/sampling | Solution or suspension | Human study not disclosed |
| MIT: SOMA | 1-5 mgs | Microneedle spike injection | Compressed sugar millipost | Preclinical insulin minipig PK data |
| Rani: RaniPill™ | 1-3 mgs | Microneedle patch injection | Sugar based microneedle | Phase 1 Safety/PK Octreotide BA 70% |
